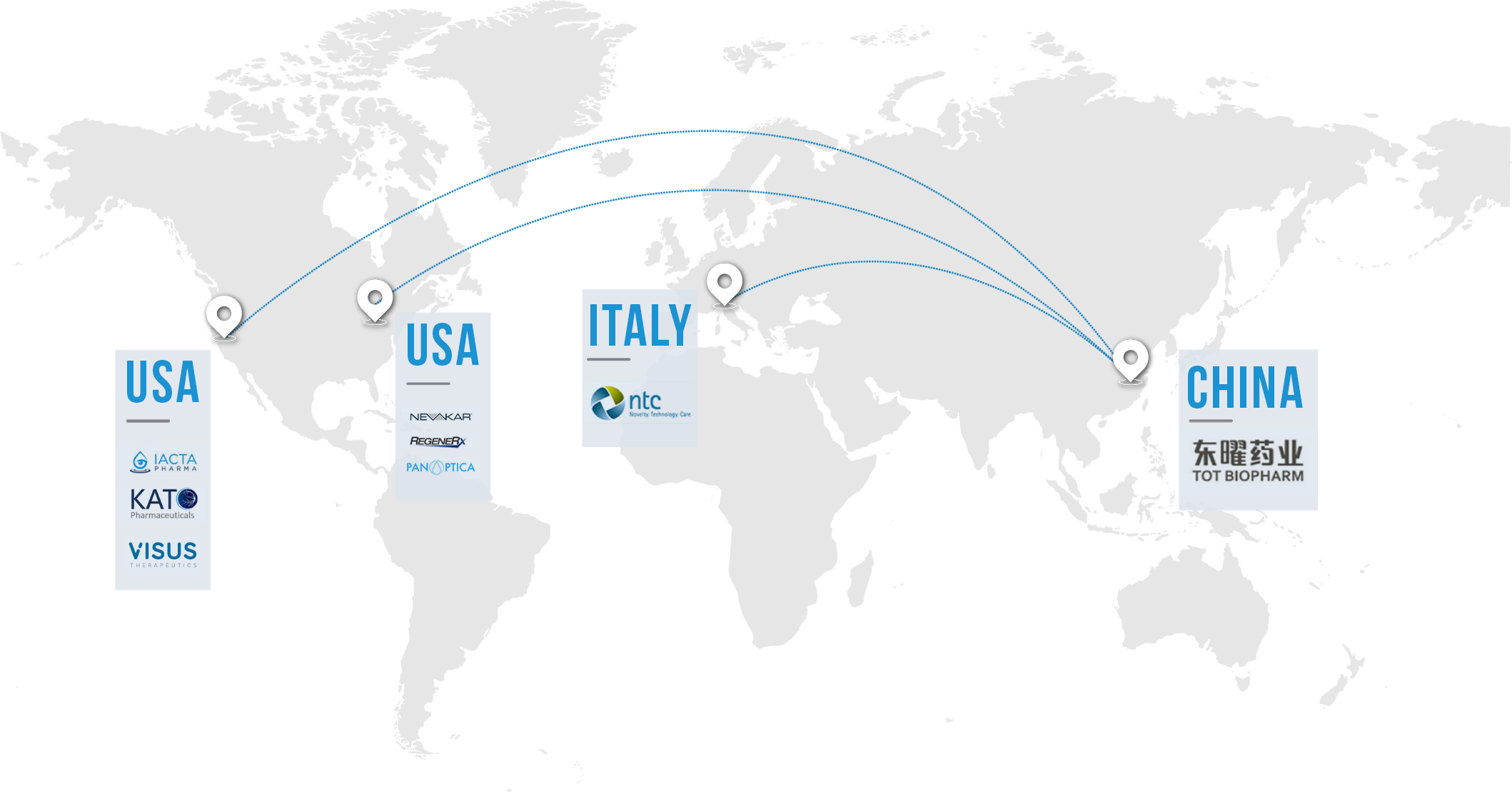
Strategic Partnerships
Strategic Partnerships












Contact Us





