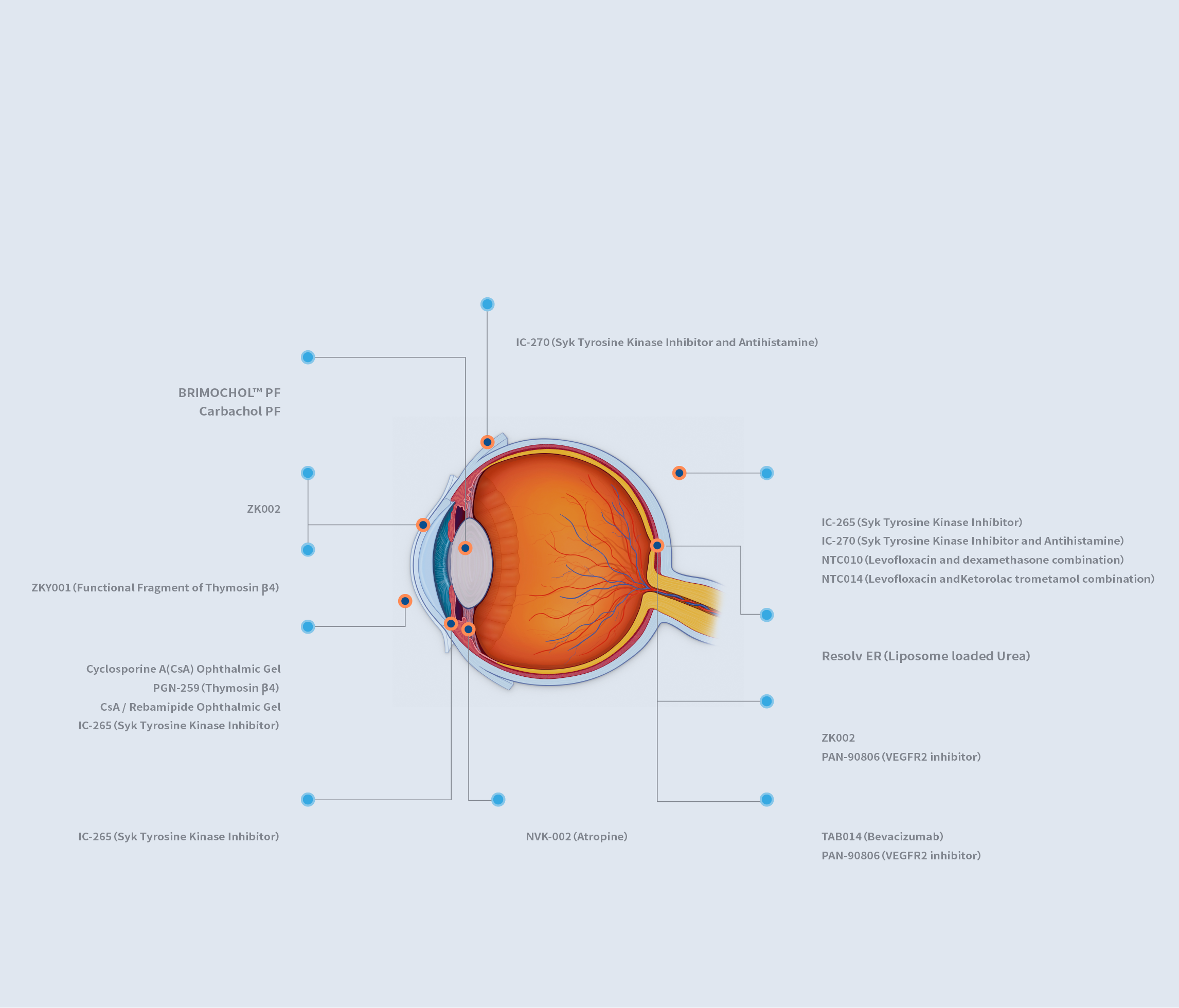
ZKY001
One of our Core Products and a eye drop targeting corneal epithelial defects, or CED, through anti-inflammatory effects plus stimulation of epithelial cell migration. Compared to widely prescribed growth factor therapies, such as rh-EGF and rb-bFGF drugs, which stimulate angiogenesis and may cause edema and inflammation, ZKY001 showed better in vivo efficacy in reducing corneal swelling and suppressing abnormal ocular vessel growth in preclinical animal models. ZKY001 also has a favorable safety profile, well tolerated at all concentrations in one of our Phase I clinical trials. We believe ZKY001 has the potential to be a foundational therapy for a broad range of corneal epithelial diseases.
We have completed a Phase I clinical trial for the safety,tolerabillty and systemic pharmacokinetics of ZKY001. ZKY001 was well tolerated or all concentrations during the trial. Among the 34 subjects who received ZKY001, only three mild AEs were reported, including two incidents of elevated blood triglyceride level and one incident of increased white blood cells in urine, and no subject experienced treatment-related AE.
We also completed two preclinical studies to evaluate the efficacy of ZKY001. The first study compared the efficacy of ZKY001 against rh-EGF, levofloxacin lactate and saline on the treatment of CED after laminectomy, a surgery which removes a layer in the cornea and, inevitably, creates damages to the cornea. ZKY001 showed faster onset effects than rh-EGF and levofloxacin lactate. In comparison to the saline group, the healing of the corneas treated with ZKY001 (measured by decrease in the proportion of damaged epithelial cells) reached statistical significance 24 hours after the surgery (p=0.005, p<0.05), whereas healing of the corneas treated with rh-EGF (p=0.004, p<0.05) and levofloxacin lactate (p=0.001, p<0.05) reached statistical significance on day 3 and day 4 after surgery, respectively. On day 7, corneas treated with ZKY001 were completely healed, whereas some corneas treated with levofloxacin lactate and saline remained unhealed.
The second study compared the efficacy of ZKY001 at three concentrations against rh-EGF and saline on the repair of CED after corneal alkali burns, serious damages to the cornea after contact with alkaline chemicals. The study showed that cell migration in the ZKY001 treatment groups was significantly higher than that in the control groups, with 20 μg/ml ZKY001 and 40 μg/ml ZKY001 being more efficacious than the other groups. In addition, 20 μg/ml ZKY001 and 40 μg/ml ZKY001 significantly alleviated corneal opacity and edema, which are evidence for corneal healing.
We started a Phase II clinical trial in November 2020 to evaluate the efficacy and safety of ZKY001 and expect to complete the trial in the fourth quarter of 2021. We plan to initiate a Phase III clinical trial in the second half of 2022 and target to submit an NDA to the NMPA in 2024.